Mouse TLR1-9 Agonist kit
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Mouse TLR1-9 Agonist kit (9 ligands) 9 ligands for murine TLR1 to TLR9 |
Show product |
1 kit |
tlrl-kit1mw
|
|
9 ligands for murine TLR1 to TLR9
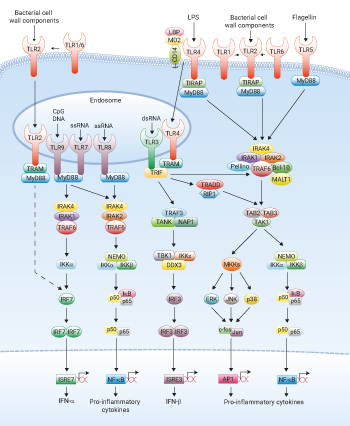
Toll-like receptors (TLRs) are pattern recognition receptors that recognize various microbial and viral molecules known as pathogen-associated molecular patterns (PAMPs).
This TLR agonist kit represents a convenient and economical tool to study the stimulation of TLRs.
This kit contains several known TLR agonists; Pam3CSK4, HKLM, FSL-1, Poly(I:C) HMW, Poly(I:C) LMW, LPS-EK, FLA-ST, ssRNA40/LyoVec™, and ODN 1826. It allows to perform up to 100 tests (in a 96-well plate).
These TLR ligands are biologically validated using HEK-Blue™ Reporter Cells.
![]() Read our review about TLR3 agonists.
Read our review about TLR3 agonists.
![]() Read our review about TLR7 and TLR8 agonists.
Read our review about TLR7 and TLR8 agonists.
![]() Read our review about TLR9 agonists.
Read our review about TLR9 agonists.
Back to the top
Contents
- TLR1/2 Agonist: Pam3CSK4 (10 µg)
- TLR2 Agonist: HKLM (109 cells)
- TLR3 Agonist: Poly(I:C) (HMW) (500 µg)
- TLR3 Agonist: Poly(I:C) (LMW) (500 µg)
- TLR4 Agonist: LPS-EK standard (LPS E. coli K12) (100 µg)
- TLR5 Agonist: FLA -ST standard (Flagellin S.typhimurium) (10 µg)
- TLR6/2 Agonist: FSL1 (10 µg)
- TLR7 Agonist: ssRNA40/LyoVec (25 µg)
- TLR9 Agonist: ODN1826 (100 µg)
- 2 x 1.5 ml endotoxin-free water
![]() Products are shipped at room temperature
Products are shipped at room temperature
![]() Products should be stored at 4°C or -20°C according to the product label.
Products should be stored at 4°C or -20°C according to the product label.
Details
• Pam3CSK4 - TLR1/TLR2 agonist Pam3CSK4 is a synthetic tri-palmitoylated lipopeptide that mimics the acylated amino terminus of bacterial lipoproteins. Pam3CysSerLys4 (Pam3CSK4) is a potent activator of the pro-inflammatory transcription factor NF-κB [1]. Recognition of Pam3CSK4 is mediated by TLR2 which cooperates with TLR1 through their cytoplasmic domain to induce the signaling cascade leading to the activation of NF-κB [2].
Molecular weight: 1852.33 g/mol
• HKLM - TLR2 agonist HKLM is a freeze-dried heat-killed preparation of Listeria monocytogenes (LM), a facultative intracellular Gram-positive bacterium. Infection with LM induces a strong non-specific response characterized by the secretion of pro-inflammatory cytokines. This response is mediated by TLR2 [3]. Stimulation with HKLM induces immediate activation of NF-κB and the production of pro‑inflammatory cytokines [4].
• FSL-1 - TLR2/TLR6 agonist FSL-1 (Pam2CGDPKHPKSF) is a synthetic lipoprotein that represents the N-terminal part of the 44-kDa lipoprotein LP44 of Mycoplasma salivarium [5]. The framework structure of FSL-1 is the same as that of MALP-2, a Mycoplasma fermentans-derived lipopeptide (LP), but they differ in the amino acid sequence and length of the peptide portion [6]. FSL-1 is recognized by TLR2 and TLR6 inducing a MyD88-dependent signaling cascade that leads to the activation of NF-κB and the production of pro-inflammatory cytokines.
Molecular weight: 1666.2 g/mol
• Poly(I:C) HMW and Poly(I:C) LMW - TLR3 agonists Poly(I:C) is a synthetic analog of double-stranded RNA (dsRNA), a molecular pattern associated with viral infection. Poly(I:C) is composed of a strand of poly(I) annealed to a strand of poly(C). The size of the strands varies. Poly(I:C) HMW has a high molecular weight (average size 1.5-8 kb), whereas Poly(I:C) LMW has a low molecular weight (average size 0.2-1 kb). Poly(I:C) HMW and Poly(I:C) LMW may activate the immune system differently. dsRNA is known to induce interferons (IFNs) and other cytokines production. IFN induction is mediated by two different pathways. The first pathway leading to NF-κB activation depends on the dsRNA‑responsive protein kinase (PKR) [7], whereas the second pathway is PKR-independent and involves TLR3 [8].
• LPS-EK (LPS from E. coli K12) - TLR4 agonist Lipopolysaccharide (LPS), the major structural component of the outer wall of Gram-negative bacteria, is a potent activator of the immune system. LPS recognition is mediated by TLR4 which forms a complex with MD2 and CD14 [9] leading to the production of pro-inflammatory cytokines through the MyD88 pathway. LPS signaling also involves a MyD88-independent cascade that mediates the expression of IFN-inducible genes via the adaptor protein TRIF [10].
• FLA-ST (Flagellin from S. typhimurium) - TLR5 agonist Flagellin is the major component of the bacterial flagellar filament, which confers motility on a wide range of bacterial species. Flagellin is a potent stimulator of innate immune responses in eukaryotic cells and organisms, including mammals and plants. In mammals, flagellin is recognized by TLR5 [11] and triggers defense responses both systemically and at epithelial surfaces. Flagellin induces the activation of NF-κB and the production of cytokines and nitric oxide depending on the nature of the TLR5 signaling complex [12].
• ssRNA40/LyoVec™ - TLR8 agonist sRNA40/LyoVec™ is a 20-mer phosphothioate-protected single-stranded RNA (ssRNA) oligonucleotide containing a GU-rich sequence [13]. ssRNA40 is complexed with the cationic lipid LyoVec™ (ratio 1:2), to protect it from degradation and facilitate its uptake, and lyophilized to generate ssRNA40/LyoVec™. When complexed to cationic lipids, ssRNA can substitute for viral RNAs in inducing TNF-α and IFN-α production in peripheral blood mononuclear cells [13, 14]. ssRNA40 complexes are recognized by TLR8 in humans and TLR7 in mice.
5’-GCCCGUCUGUUGUGUGACUC-3’ (phosphorothioate bases)
• ODN 1826 - TLR9 agonist CpG ODNs are synthetic oligonucleotides containing unmethylated CpG dinucleotides in particular sequence contexts that induce strong immunostimulatory effects through the activation of TLR9 [15, 16]. ODN1826 is the optimal motif to induce the proliferation of B cells [17].
5’- tcc atg acg ttc ctg acg tt -3’ (phosphorothioate bases)
References:
1. Aliprantis A.O. et al., 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science.285(5428):736-9.
2. Ozinsky A. et al., 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. PNAS. 97(25):13766-71.
3. Flo T.H. et al., 2000. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol, 164(4):2064-9.
4. Hauf N. et al., 1997. Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-kB (RelA/p50) activation induced by lipoteichoic acid and phospholipases and mediated by IκBα and IκBβ degradation. PNAS 94(17):9394‑9.
5. Shibata K.I. et al., 2000. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J. Immunol. 165:6538–6544.
6. Okusawa T. et al., 2004. Relationship between Structures and Biological Activities of Mycoplasmal Diacylated Lipopeptides and Their Recognition by Toll-Like Receptors 2 and 6. Infect Immun. 72(3): 1657-1665.
7. Chu W.M. et al., 1999. JNK2 and IKK beta are required for activating the innate response to viral infection. Immunity, 11(6):721-31.
8. Alexopoulou L. et al., 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature, 413(6857):732-8.
9. Re F. & Strominger J.L., 2003. Separate Functional Domains of Human MD-2 Mediate Toll-Like Receptor 4-Binding and Lipopolysaccharide Responsiveness.
10. Yamamoto M. et al., 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301(5633):640-3.
11. Hayashi F. et al., 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410(6832):1099-103.
12. Mizel S.B. et al., 2003. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol. 170(12):6217-23.
13. Heil F. et al., 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 5;303(5663):1526-9.
14. Diebold S.S. et al., 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 5;303(5663):1529-31.
15. Krieg A.M. et al., 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature, 374(6522):546-9.
16. Bauer S. et al., 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. PNAS 98(16):9237-42.
17. Ballas Z.K. et al., 2001. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 167(9):4878-86.